Indicaid®
INDICAID® Covid-19 OTC Rapid At-Home Self-Test
INDICAID® Covid-19 OTC Rapid At-Home Self-Test
Impossible de charger la disponibilité du service de retrait
Product Features:
• NDC # 60008-0407-08(2-Test Pack), 60008-0407-81(12-Test Pack)
• FDA EUA Authorized: INDICAID® COVID-19 Rapid Antigen At-Home Test is authorized by the FDA for detecting SARS-CoV-2, including Delta & Omicron variants under the Emergency Use Authorization.
• Proven Track Record: Over 45 million INDICAID tests sold globally in 30+ countries.
• Shelf-Life Extension: FDA granted a three-month extension, extending the shelf-life of all INDICAID® tests from 6 months to 15 months, effective from January 11, 2023.
• Easy-to-Use: No special training or equipment needed. Four simple steps provide accurate results in just 20 minutes.
• Accurate for Ages 2+: Suitable for asymptomatic or symptomatic individuals aged 2 and above. Children aged 2-13 should be tested by an adult.
• Recommendation for Asymptomatic Individuals: If asymptomatic and the result is 'negative,' a second test is suggested between 24 to 48 hours after the initial test.
• Versatile Application: Perfect for travel, school, work, and gatherings. Offers quick results for peace of mind in possible exposure situations.
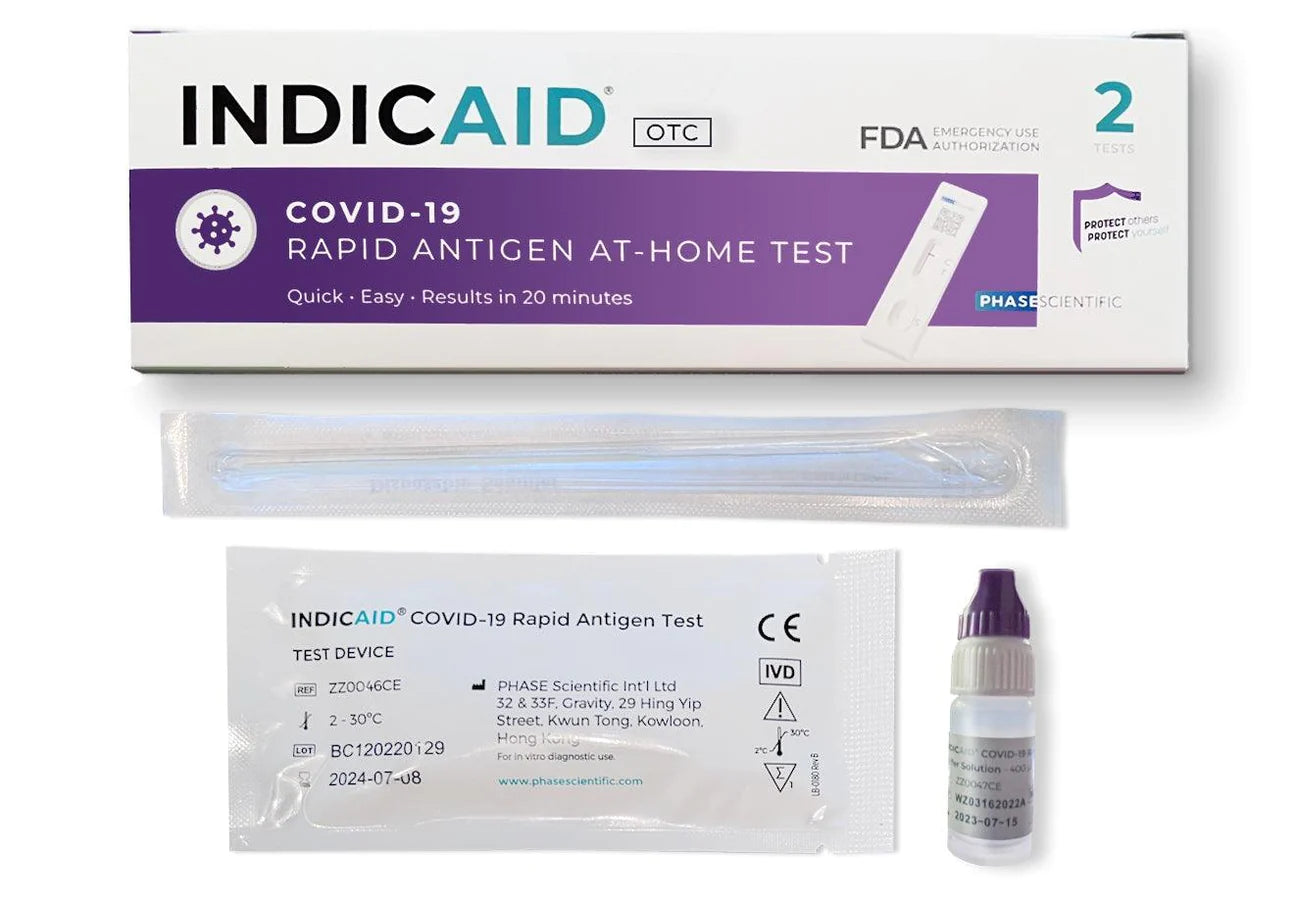
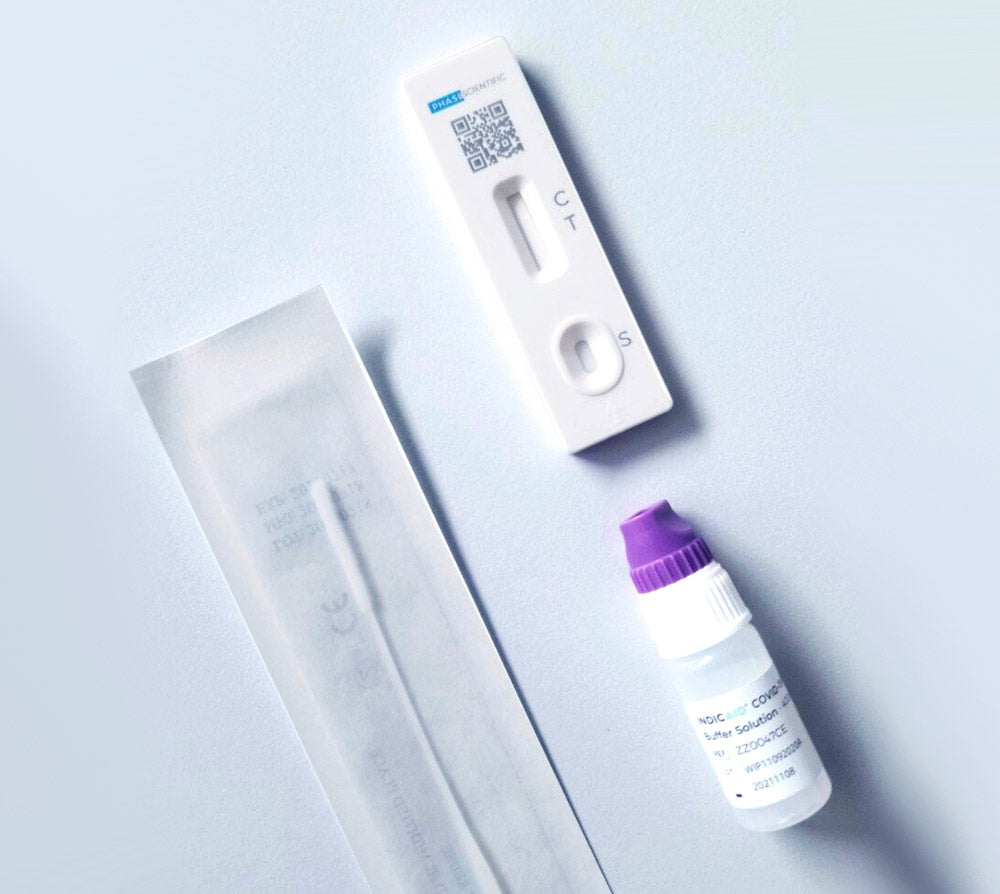
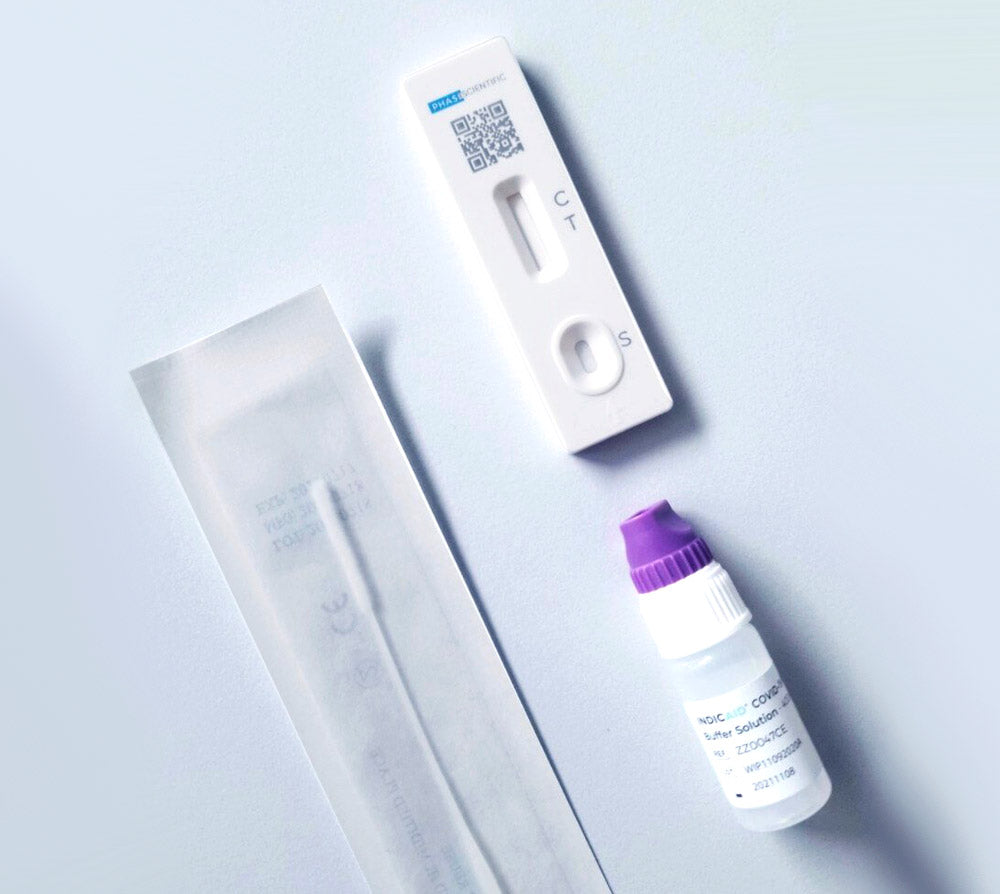
• Comprehensive Kit: The INDICAID® OTC COVID-19 Rapid Antigen At-Home Test (2-Tests) includes 2 sterile nasal swabs, 2 buffer solution vials, 2 test devices (Cartridges), a quick reference guide, and user instructions.
Each 2-Pack Contains:
• 2 Individually wrapped Sterile Nasal Swabs
• 2 Single-use Buffer Solution Vials
• 2 Individually packaged single-use Test Devices (Cartridges)
• 1 Quick Reference Guide
• 1 User instructions
Disclaimer:
The INDICAID® COVID-19 Rapid Antigen Test is intended for non-prescription at-home self-test and/or as applicable an adult lay user testing another person 2 years of age or older in a non-laboratory setting. The INDICAID® COVID-19 Rapid Antigen Test is only for invitro diagnostic use for use under the Food and Drug Administration's Emergency Use Authorization. This product has not been FDA cleared or approved.
Resources:
• INDICAID COVID-19 Rapid Antigen At-Home Test - Letter of Authorization (shopify.com)
• INDICAID COVID-19 Rapid Antigen At-Home Test - Instructions for Use Home Testing (shopify.com)
• sds-indicaid-otc-0034aSDS_rev-b.pdf (pressidium.com) – SDS Sheet
Pro1Tek is an authorized, direct distributor of Phase Scientific.
Description:
The INDICAID® COVID-19 Rapid Antigen At-Home Test is an FDA EUA authorized powerful tool designed for detecting SARS-CoV-2, the virus responsible for COVID-19. This test can also detect the Delta and Omicron variants. With a global reach of over 45 million tests sold in 30+ countries, the INDICAID test has proven its reliability. Notably, the FDA granted a three-month shelf-life extension on January 11, 2023, extending the shelf-life from 6 months to 15 months for all INDICAID® COVID-19 Antigen Rapid Tests, as detailed on the FDA website.
This test is user-friendly, rapid, and pain-free, requiring no special training or equipment. With just four easy steps, including a gentle swab of the lower nasal cavity, stirring the swab in the provided solution, and pouring drops onto the testing device, accurate results are obtained within 20 minutes. The INDICAID® test is versatile, accurately testing individuals aged 2 and above, whether asymptomatic or symptomatic. If asymptomatic and the result is 'negative,' a second test is recommended between 24 to 48 hours after the initial one. It's a perfect solution for various settings, including travel, school, work, and gatherings, offering peace of mind with quick results.
INDICAID® is a fast, reliable, and affordable COVID-19 Rapid Antigen At-Home Test for use by your patients, customers, and employees wanting to know if they may have contracted COVID-19, by living their daily lives.
FEATURES SPECIFICATIONS
PRODUCT CODE EUA220152
ITEMS PER BOX 2, 12
HSA/FSA ELIGIBLE
SPECIMAN TYPE Direct Anterior Nasal swab
INTENDED USE The INDICAID® COVID-19 Rapid Antigen At-Home Test is intended for non-prescription self-use and/or as applicable an adult lay user testing another person 2 years of age or older. The INDICAID® COVID-19 Rapid Antigen At-Home Test is only for use under the Food and Drug Administration’s Emergency Use Authorization.
STORAGE CONDITION 36º-86ºF (2º-30ºC) Do not freeze. Avoid direct sunlight.
SHELF LIFE 12 Months
ACCURACY Sensitivity 81.7%; Specificity 99.4%; Overall accuracy 93.4%
Partager